The Institute of Physics of the Chinese Academy of Sciences and the Songshan Lake Materials Laboratory have made new progress in the research of lithium ion battery materials based on the Chinese spallation neutron source.
Lithium-ion batteries have been used as the technology of choice in the field of energy storage because of their advantages in various aspects. Continuously improving its energy density has been the direction of efforts of scientists and technicians from various countries. The energy density of a lithium ion battery is positively related to the amount of lithium intercalated per unit mass of the cathode material. Many studies have shown that the lattice oxygen in the lithium-rich oxide cathode material can greatly increase the amount of lithium intercalated by the material through its own valence (redox) reaction, thereby achieving a higher energy density. However, how to design the structure of the control material to achieve a stable and reversible oxygen valence reaction has not been very clear.
Recently, Dr. Zhao Enyue and Dr. Li Qinghao, PhD students from Institute of Physics, Chinese Academy of Sciences / Beijing National Research Center for Condensed Matter Physics, under the joint guidance of dual-researchers Wang Fangwei and Yu Xiqian from Songshan Lake Materials Laboratory, together with Dr. Meng Fanqi and Researcher Gu Lin , He Lunhua, Wanli Yang, a researcher at the Lawrence Berkeley National Laboratory in the United States, and researchers from the Shanghai Synchrotron Radiation Light Source, the Oak Ridge National Laboratory in the United States, and the Spallation Neutron Source in Japan. Based on the results (Energy Storage Materials 16 (2019) 354), the first combination of neutron powder diffraction and neutron pair distribution function technology reveals that the 3D disordered cation framework structure can be used to stabilize oxygen in lithium-rich oxide cathode materials The lattice and oxygen change in value. The researchers found that unlike the distorted oxygen lattice of traditional layered lithium-rich materials (2D cationic ordered structure), lithium-rich materials with a 3D cationic disordered structure can maintain relatively stable oxygen when the lattice oxygen undergoes a valence reaction Lattice skeleton. This difference in oxygen lattice structure mainly results from the different structural dimensions of the two material systems. The lattice oxygen change reaction usually occurs on the unhybridized O2p orbital (Li-O-Li coordination configuration) in the lithium-rich material. In order to reduce the energy of the entire material system, the distance between the oxidized lattice oxygen ions Will shorten (that is, oxygen lattice distortion). The premise of this oxygen lattice distortion is that the two unhybridized O2p orbitals must be coplanar. However, in cation-disordered lithium-rich materials, the 3D disordered spatial distribution of cations leads to a very high probability that unhybridized O2p orbitals are not coplanar with each other. Therefore, a stable oxygen lattice structure was observed in the cationic disordered lithium-rich material. This stable oxygen lattice framework in turn promotes the reversibility of the lattice oxygen valence reaction in the material. The results of this work show that the structural dimensions of the material can be designed and adjusted to achieve the stability and reversibility of the lattice oxygen valence reaction. More importantly, the work reveals the effect of structural dimensions on the lattice oxygen valence reaction and the overall oxygen lattice structure.
This research provides a very good theoretical basis and research and development ideas for the design of subsequent high-performance new materials. The results were published in "Angel. Chem. Int. Ed." And were selected as Very Important Paper by the editorial department of the journal. (VIP, Top 5%), ChemistryViews, an international professional media, also has key reports.
This work was supported by the National Key Research and Development Program of the Ministry of Science and Technology, the National Natural Science Foundation of China, and the Songshan Lake Materials Laboratory.
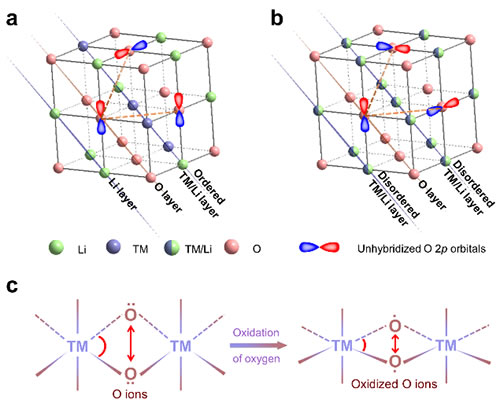
Figure 1. Schematic diagram of the lithium-rich oxide cathode material structure.
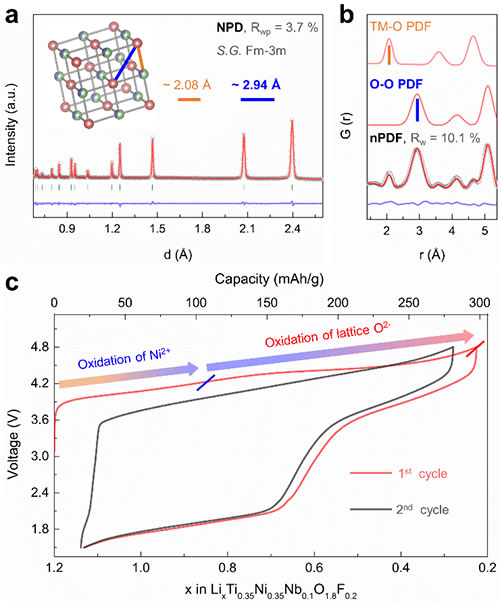
Figure 2. Material neutron powder diffraction, neutron pair distribution function, and electrochemical data.
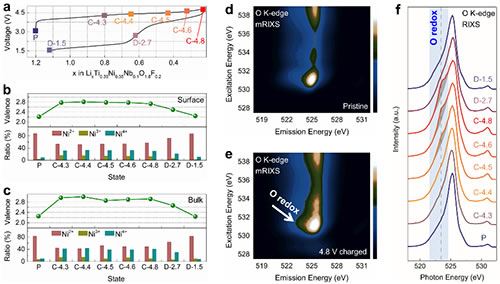
Figure 3. The process of charge compensation for oxygen valence of materials.
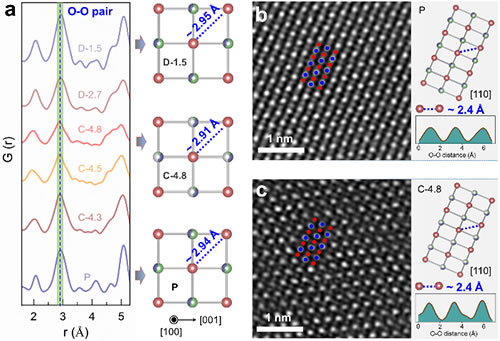
Figure 4. Evolution of the oxygen lattice structure during the oxygen valence process.
An electric Butterfly Valve is a type of valve that uses an electric motor to control the opening and closing of the valve. The valve consists of a disc that rotates around a central axis to control the flow of fluid or gas. When the electric motor is activated, it rotates the disc to open or close the valve. Electric butterfly valves are commonly used in industrial applications such as water treatment, chemical processing, and HVAC systems. They are preferred over manual valves because they offer precise control and can be operated remotely.
Electric Butterfly Valve,Electric Actuated Butterfly Valve,Electric Butterfly Valve Price,Electric Butterfly Valve
WUXI KVC-VALVE , https://www.wxkaiweixi.com