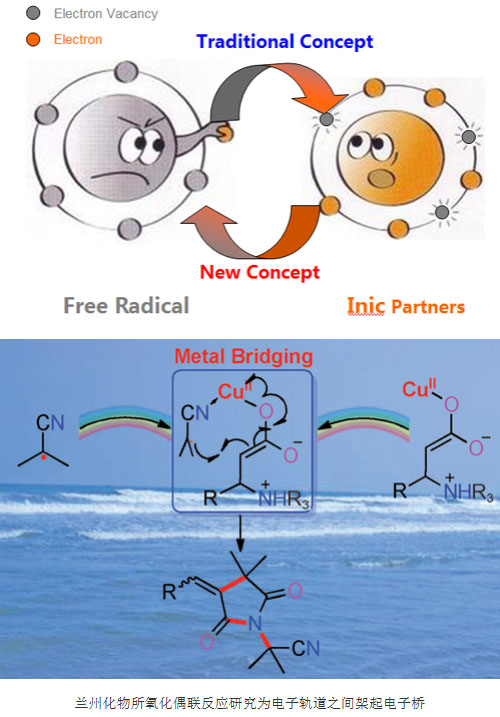
Electron transfer is the cornerstone and precondition for the occurrence of chemical reactions. Electron transfer can only occur between orbits with equivalent energy levels. The energy levels between orbits are too different and electrons are difficult to transfer. Chemical reactions naturally occur. Can not happen. “Bridge†is ubiquitous in our real life. It allows people on both sides of the chasm to freely pass through and communicate, and brings people closer together. If we can learn from the concept of the macroscopic world and set up an “electronic bridge†between the orbits of different energy levels, it is possible to realize the electronic transfer difficult to achieve by conventional methods and construct some new organic chemical reactions.
Based on this idea, the research team led by Huang Hanmin, a researcher at the Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences, has worked hard for nearly four years to build a copper “electronic bridge†between free radicals and polar compounds, realizing free radicals and The single-electron transfer between polar ionic compounds has led to the creation of an oxidative coupling reaction for single-electron transfer of free radicals. The results of this study were published online using Metal Bridging for Directing and Accelerating Electron Transfer as Exemplified by Harnessing the Reactivity of AIBN. In Angew. Chem. Int. Ed. (Angew. Chem. Int. Ed. 2015, 54, DOI: 10.1002/anie.201411974).
It is well-known that free radicals are a common reactive species. Although their reactivity is high, their selectivity is difficult to control. Therefore, how to achieve single-electron transfer of free radicals has always been a problem in the field of free radical chemistry research. In the free radical reaction in which transition metals participate, the role of the metal is generally to generate free radicals or convert the free radicals into corresponding ionic species to achieve the directional transfer of their single electrons. This group used this strategy to realize the direct use of toluene. Carbonylation and the corresponding single-electron coupling reaction (J. Am. Chem. Soc. 2012, 134, 9902-9905; Org. Lett. 2013, 15, 3370-3373; ACS Catal. 2015, 5, 2882-2885) .
In this strategy, since the activity of free radicals is generally high, the single electron transfer generally flows from free radicals to other reactive species. Obviously, if one can change free radicals into electron acceptors to make them more inclined to accept electrons, then the selectivity of the reaction can be effectively controlled and organic chemical reactions that are difficult to achieve with conventional methods can be realized. An effective strategy is to introduce a suitable coordinating group into the free radicals, which reduces the energy of the free radical SOMO orbital by the coordination of the transition metal and the coordination group, making it tend to accept electrons. This will be The activation of free radicals provides a new idea, but it is not easy to achieve the above concepts.
AIBN is one of the most commonly used azo initiators. In addition to being a foaming agent for plastics and rubbers, AIBN is best known as a free radical initiator. AIBN can be decomposed very smoothly and only one kind of radical is obtained, and it is basically not further induced to decompose, but the isobutyronitrile radical produced by it is poor in electrophilicity and nucleophilicity, and it is almost not used as a reactant. A precedent for organic synthesis. The researchers used Cu as a catalyst to coordinate the metal with the cyano group of the free radical and the corresponding nucleophile. An electron bridge was built between the two reactants through the metal, accelerating and guiding the transfer of electrons. A new strategy for radical activation has been achieved, and the oxidative coupling-cyclization reaction of inert AIBN and cinnamic acid has been achieved.
Theoretical calculations and electron paramagnetic resonance (EPR) and control experiments confirm the dual role of catalyst copper: on the one hand, the free radical SOMO orbital energy is reduced by coordination, free radicals are activated, and on the other hand act as electron bridges to make free radicals and nucleophiles. The reagents move closer to each other to accelerate electron transfer. This strategy achieves the transition of the isobutyronitrile radical from the initiator to the reaction substrate. Through this strategy, a highly functional and physiologically active 2 is efficiently synthesized starting from the easily available AIBN and cinnamic acid. 5-pyrrolidinedione. This research work has successfully constructed a new model of oxidative coupling reaction for reverse electron transfer, establishing a new concept for the reaction chemistry of free radicals, which is bound to promote the development of free radical chemistry.
The above work has received long-term support from the National Natural Science Foundation of China and the Lanzhou Institute of Chemical Industry's "One-Three-Five" Plan for Key Fostering Projects.
Recessed Led Pool Light,Underwater Pool Light Fixture,316 Recessed Pool Light,Waterproof Recessed Swimming Lights
Guangzhou Qshine Pool Lights Manufacture Co., Ltd , https://www.qshinepoollight.com