Methane, which is the main component of natural gas and shale gas, has the advantages of relatively abundant reserves and low prices. It is one of the cores of research and development in academia and industry in the field of replacing petroleum to produce liquid fuels and basic chemicals. Oxygen derivatives of methane, especially alcohol derivatives, are considered to be the backbone of carbon-chemistry; while methane is the most stable small organic molecule, after the CH bond is activated, it obtains a highly active intermediate species, which is prone to excessive activation and Completely mineralized. Because of the broad prospects and huge challenges, the selective activation and directional conversion of methane is a worldwide problem, known as the "Holy Grail" in the field of catalysis and even chemistry. So far, the conversion of methane is usually indirect: by steam reforming at high temperature to convert methane to synthesis gas, and then by Fischer-Tropsch synthesis to obtain multi-carbon basic chemicals; or syngas to produce methanol, and then produce other chemicals . The conversion route is lengthy and consumes a lot of energy. In the process, a large amount of greenhouse gas carbon dioxide is emitted, which not only brings environmental load, but also makes the total carbon utilization rate less than half. Therefore, scientists have been working hard to explore methods for the direct conversion and utilization of methane.
Direct photocatalytic conversion can break the shackles of traditional thermodynamic equilibrium, so that the conversion of methane can be carried out at low temperature and normal pressure. Recently, the research team led by Wang Wenzhong, a researcher at the Shanghai Institute of Ceramics, Chinese Academy of Sciences, has made new progress in the research of photocatalytic conversion of methane. The team designed and prepared copper-modified carbon nitride materials to achieve the photocatalytic direct conversion of methane to ethanol, and conducted a more in-depth study of the mechanism of the process. The relevant research results were published in "Nature Communications" (Nature Communications, DOI: 10.1038 / s41467-019-08454-0) with the theme of Direct functionalization of methane into ethanol over copper modified polymeric carbon nitride via photocatalysis, and applied for a Chinese invention patent One (201811339733.2), the first author is Zhou Yuanyi, a doctoral student of Shanghai Silicate Institute.
In view of the problem that methane is prone to over-activation and complete mineralization, from the perspective of the generation of reactive oxygen species and the adsorption and activation of methane, the research team not only achieved the realization of copper modification in the ordered cavity of the carbon nitride material The in situ generation of hydroxyl radicals also promotes the activation of methane CH bonds and the stability of highly active intermediate species. The material exhibits excellent photocatalytic methane conversion performance, and the ethanol yield reaches 106 μmol g-1 h-1, which is the best value reported in related fields. In-depth studies have shown that in addition to the free radical mechanism, the copper species in this material have a synergistic effect with the adjacent carbon atoms, making the conversion process follow the methane-methanol-ethanol path. This work proposes a new strategy for the direct conversion of methane to liquid fuel under mild conditions, which helps to deepen the understanding of the formation mechanism of multi-carbon products.
Relevant research work is supported and supported by the National Natural Science Foundation of China.
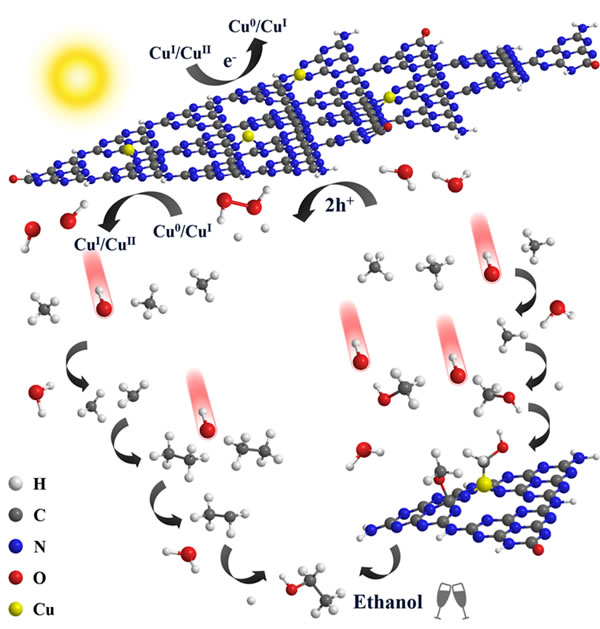
High-efficiency photocatalytic direct methane conversion based on copper modified carbon nitride
Microscope Accessories ,Microscope Eyepiece,Microscope Camera Adapter,Microscope Slides And Cover Slips
Ningbo Beilun Kalinu Optoelectronic Technology Co.,Ltd , https://www.nbyxmicroscope.com